
Shares of Bristol-Myers Squibb Company (BMY) increased nearly +20% during the time period surrounding the American Society of Clinical Oncology’s (ASCO) 2013 Annual Meeting. The day after the meeting abstracts were made public on May 15, shares were trading up +8% from the start of the month and would further rise more than +11% by the last day of the conference on June 3 (Chart 1). With approximately 1.64 billion shares outstanding, the rise in stock price during this period added $12.9 billion to Bristol-Myers’ market capitalization.
Chart 1:
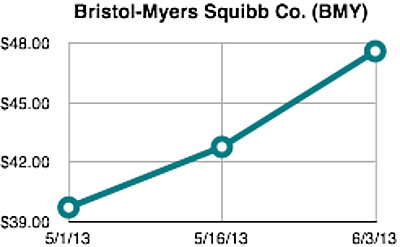
This type of dramatic stock price movement in such a short time period is more characteristic of small-capitalization biotechnology companies than large pharmaceutical firms. However, investor interest in Bristol-Myers was driven by increasing recognition about the disruptive potential of immunotherapy—using your body’s own immune system—to treat cancer and many other diseases.
Indeed, a research report titled “Immunotherapy—The Beginning of the End for Cancer” issued by analysts at U.S. bank Citigroup on May 22, 2013, boldly predicts that the annual market for immuno-therapies—defined as including checkpoint agents, vaccines and cell therapy—will exceed $35 billion and become the backbone of treatment in up to 60% of cancers over the next decade.
Three blockbuster monoclonal antibody (mAb) products currently sold by the Roche Group (ROG.VX, RHHBY)—Avastin® (bevacizumab), Rituxan® (rituximab), and Herceptin® (trastuzumab)—collectively represented more than US$19.4 billion in annual revenue for the compa-ny in 2012.[i] As a result, the recently published estimate of $20 billion in potential sales for various checkpoint inhibitor mAbs alone within the next decade does not appear unrealistic.
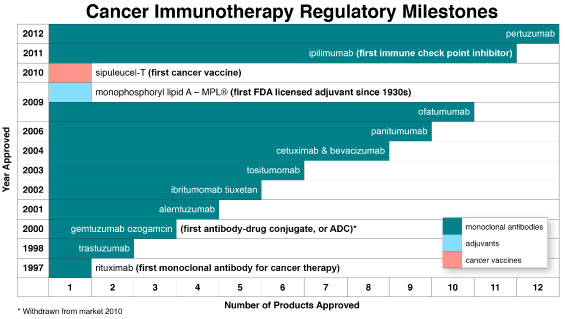
When viewed in the context of passive immunization, active immunization, and immunostimula-tion, cancer immunotherapy has provided hope to patients, physicians, and others as a promising new treatment modality with limited side effects and superior efficacy dating back to November 1997 when the first mAb for cancer therapy, Rituxan® (rituximab) by Biogen Idec Inc. (BIIB), was approved by the U.S. Food and Drug Administration (FDA). As evidenced in Chart 2, the field of cancer immu-notherapy has since achieved a number of additional regulatory milestones, including FDA approval of eleven additional mAbs, the first cancer vaccine—Provenge® (sipuleucel-T) by Dendreon Corpora-tion (DNDN), and the first licensed vaccine adjuvant since the 1930s—MPL® (mono-phosphoryl lipid A) by GlaxoSmithKline plc (GSK). Included among the dozen approved mAbs are the first antibody-drug conjugate and, more recently, the first immune checkpoint inhibitor.
Checkpoint Inhibitors
The body’s immune system has a braking mechanism, a “checkpoint” to prevent immune responses to self-markers. Without this checkpoint, autoimmune disease could flourish. Much of the recent excitement in cancer immunotherapy has been generated by the promising clinical data with human mAbs that bind to certain checkpoint proteins, such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1). Blocking these immune checkpoint proteins disables a brake that prevents the immune system from attacking cancer cells and has produced promising clinical data in various tumor types, such as melanoma, renal cell carcinoma and non-small cell lung cancer (NSCLC).
FDA approval of the first-in-class product (Bristol-Myers Squibb’s Yervoy®, ipilimumab) for mela-noma occurred in 2011. Yervoy generated $229 million in worldwide sales during the first quarter of 2013,[ii] which would translate to $916 million on an annualized basis. By releasing a brake from the body’s immune system, Yervoy causes a broad range of immune-related adverse events, some of which can be serious or fatal.
During ASCO’s 2013 Annual Meeting, Bristol-Myers Squibb, Merck & Co. Inc. (MRK), and Genentech—a member of the Roche Group—presented updated and new data from their next-generation checkpoint inhibitors directed against PD-1 or PD-L1. This includes Bristol-Myers’ nivolumab and Merck’s lambrolizumab (or MK-3475), which are both PD-1 inhibitors, and Roche’s PD-L1 inhibitor called MPDL3280A. These next-generation checkpoint inhibitors appear to offer better efficacy and lower toxicity when compared to Yervoy.
Although too early to tell for certain, Merck’s lambrolizumab appears to be among the most prom-ising agents in advanced melanoma patients, with a confirmed response rate of 52% at the highest dose level.[iii] This compares favorably with the 53% objective-response rate demonstrated by the maximum tolerated concurrent doses of Bristol-Myers’ two checkpoint inhibitors—Yervoy and nivolumab—in this disease setting.[iv] However, Bristol-Myers’ nivolumab product is in later-stage development and will likely be first to market.
Due to the potential for immune-related side effects, checkpoint inhibitors have largely been stud-ied in cancer patients with late-stage disease. Accordingly, there is a large unmet medical need for cancer patients with less advanced disease, which could be addressed by the next wave of cancer vaccines.
Cancer Vaccines
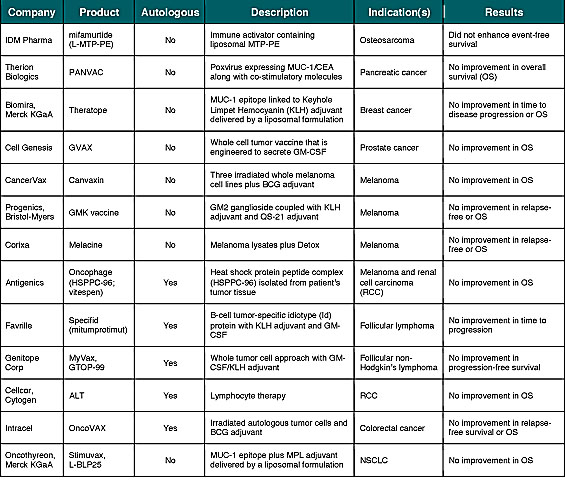
With few exceptions, enthusiasm for checkpoint inhibitors and other mAbs hasn’t translated into investor interest in cancer vaccines—at least not yet. This is due in part to a high degree of investor skepticism following the failure of more than a dozen cancer vaccines in late-stage (phase 3) devel-opment (Table 1). However, in a recent telephone interview, James L. Gulley, M.D., Ph.D., F.A.C.P., of the Center for Cancer Research at the National Cancer Institute (NCI) noted that he has seen an actual increase in cancer vaccine interest by big pharma companies. Dr. Gulley added that there is much more enthusiasm than there was a year ago, as everybody is scrambling to find rational agents to combine with their immune checkpoint inhibitors.
In general, the failure of many cancer vaccines can be explained through one or more of the fol-lowing factors:
1) Disease stage: Studying the cancer vaccine in late-stage patients (or those with bulky disease burden) as opposed to earlier-stage patients (or those with minimal residual disease after primary therapy). In fact, several of the vaccines listed in Table 1 demonstrated encouraging results in subsets of earlier-stage patient populations. This includes Antigenics’ predefined exploratory analyses of Oncophage in earlier-stage RCC patients[v] and the M1a and M1b subsets of Stage IV melanoma[vi], along with Intracel’s On-coVAX in Stage II colorectal cancer.[vii]
2) Immune force: Every cell in your body carries the same set of distinctive surface proteins that distinguish you as “self” and any “non-self” substance capable of triggering an immune response is known as an antigen.[viii] Some early cancer vaccines incorporated suboptimal antigen targets and/or lacked requisite adjuvant(s), or immune stimulants, that made it difficult to overcome immune tolerance.
3) Big pharma support: Drugs that succeed in phase 3 clinical trials tend to be developed by larger companies with strong records of accomplishment in drug development and/or the resources to run large, expensive studies. While not specific to the field of immunotherapy, one published analysis concluded that there have been no positive phase 3 trials among companies with less than $300 million in market capitalization at 120 days before public disclosure of trial results—known as the Feuerstein-Ratain rule.[ix] Historically, cancer vaccines have been developed by smaller biotechnology companies, many of which were likely below the $300 million market capitalization cutoff under the Feuerstein-Ratain rule.
Looking ahead over the coming months, there is reason for optimism regarding the next wave of phase 3 results from ongoing cancer vaccine trials. This is due to the fact that many of these products address the limitations of prior cancer vaccine approaches and/or have support from big pharma.
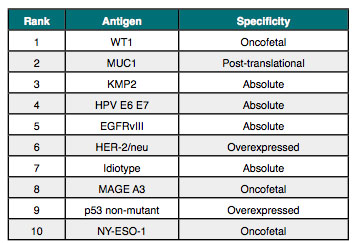
For example, GlaxoSmithKline is undertaking two large studies with the company’s MAGE-A3 Antigen Specific Cancer Immunotherapeutic (ASCI) and is likely the next major catalyst for the cancer vaccine field, with phase 3 data expected to be delivered during 2013 and 2014.[x] As depicted in Table 2, MAGE-A3 is ranked among the top ten according to an antigen prioritization project sponsored by the NCI.[xi] The ranking, which included 75 different antigens, was based on the likelihood for efficacy in cancer therapy and was ex-ceedingly well vetted, with broad and substantial input from academia, industry, and the govern-ment.
GlaxoSmithKline’s MAGE-A3 ASCI is further enhanced through the use of AS15—an adjuvant system. AS15 is a liposomal formulation of CpG, MPL and the QS-21 Stimulon® adjuvant licensed from corporate partner Agenus, Inc. (AGEN). GlaxoSmithKline’s various adjuvant systems have been shown to offer an improved quality of the immune response by inducing a more enhanced, targeted stimulation of the immune response to produce strong, sustained and broad protection. Through the use of these novel adjuvant systems, several of GlaxoSmithKline’s vaccines have demonstrated protection against challenging diseases formerly thought to be beyond the reach of vaccination.[xii]
Table 2. Top Ten Antigens by NCI Ranking Project
Beyond incorporating both an ideal antigen and adjuvant system, GlaxoSmithKline is conducting two large phase 3 studies in patients with minimal residual disease following therapy. For example, the melanoma study enrolled 1,349 stage IIIB or IIIC cutaneous melanoma patients that must have been surgically rendered free of disease before randomization. Similarly, the NSCLC study is the largest clinical trial ever conducted in lung cancer treatment and has enrolled 2,289 patients with completely resected, pathologically proven stage IB, II or IIIA NSCLC. In both the melanoma and NSCLC studies, the patient’s tumor must also show expression of the MAGE-A3 gene.
In addition to GlaxoSmithKline, Celldex Therapeutics, Inc. (CLDX), Transgene SA (TNG.PA), NewLink Genetics Corporation (NLNK), and others are expected to report key data in 2013 and 2014. Several of these companies also have programs designed to address the limitations of prior vaccine approaches.
For example, Celldex is developing rindopepimut (CDX-110) as a cancer immunotherapy that targets EGFRvIII, another antigen ranked among the top ten according to the NCI’s antigen prioritization project. Rindopepimut is given along with GM-CSF as a vaccine adjuvant.
Rindopepimut is being studied for the treatment of glioblastoma, the most common primary ma-lignant brain tumor. Glioblastoma is a popular disease focus for several cancer vaccine developers and, more recently, a large cooperative group. Despite aggressive treatment, which includes surgical resection, local radiotherapy and systemic chemotherapy, the median survival time after diagnosis is still in the range of just 12 months.[xiii]
During 2013, Celldex expects to complete global recruitment in the phase 3 ACT IV registration study of rindopepimut in front line glioblastoma and in the phase 2 ReACT study of rindopepimut in combination with Roche’s Avastin in patients with recurrent/refractory EGFRvIII-positive glioblasto-ma.[xiv] Results from the ReACT study are expected by year-end.
In May 2013, enrollment started for another cancer vaccine (HSPPC-96) being studied in combi-nation with Avastin in the recurrent glioblastoma setting. The randomized phase 2 study is being funded by the Alliance for Clinical Trials in Oncology, an NCI cooperative group. Seeking to enroll 222 patients, this represents the largest vaccine trial ever funded by the NCI in brain tumors and the larg-est cancer vaccine study ever conducted in combination with Avastin.[xv]
Transgene is developing TG4010, which is a cancer vaccine based on a recombinant vaccinia virus expressing the MUC1 antigen and the human cytokine, Interleukin-2 (IL2). MUC1 ranks second on the list of 75 antigens prioritized by the NCI. TG4010 is meant to induce both innate and adaptive immune responses.
In March 2010, Transgene signed an exclusive option agreement with Novartis AG (NVS) for TG4010. A pivotal, global, controlled phase 2b/3 trial of TG4010 in patients with advanced (Stage IV) NSCLC started in the second quarter of 2012 and phase 2b data is expected in the second half of 2013.[xvi]
NewLink's lead product candidate, algenpantucel-L (HyperAcute pancreas), is being studied in a phase 3 trial under a Special Protocol Assessment with the FDA. This trial involves up to 722 patients with surgically resected pancreatic cancer and the first interim analysis will be conducted when 222 deaths are reported for the study. Algenpantucel-L is also being tested in a second phase 3 study involving patients with locally advanced pancreatic cancer.
Algenpantucel-L consists of a group of two allogeneic pancreatic cancer tumor cell lines that are tumor specific, but not patient specific. These cells have been modified to express alpha-gal (α-gal), a carbohydrate abundantly synthesized from glycoproteins and glycolipids in non-primate mammals and New World monkeys, but absent in humans, apes and Old World monkeys.[xvii]
Vical Incorporated (VICL) is approaching completion of a phase 3 registration trial of its investiga-tional immunotherapy, Allovectin-7®, versus chemotherapy in patients with confirmed Stage III or Stage IV melanoma. Top-line results for both the primary endpoint (response rate at 24 weeks or more after randomization) and secondary endpoint (overall survival) are expected to be released during the third quarter of 2013.[xviii]
Administered as an intralesional injection, Allovectin-7 is designed to stimulate both innate and adaptive immune responses in local tumors and distal metastases. It is a plasmid-based immunotherapeutic expressing two genes (HLA-B7 and β2 microglobulin) that together form an MHC class 1 complex.
GlaxoSmithKline, Celldex, Transgene, NewLink and Vical each have current market capitaliza-tions above the $300 million cutoff for the Feuerstein-Ratain rule, potentially signaling investor confidence in their respective cancer vaccine programs. The outliers in this regard are ImmunoCellular Therapeutics, Ltd. (IMUC) and Northwest Biotherapeutics, Inc. (NWBO), both of which have current market capitalizations around $100 million and are developing autologous cancer vaccines.
A Data Monitoring Committee (DMC) recently completed a pre-specified interim analysis of ImmunoCellular’s ICT-107, a 6-antigen dendritic cell vaccine targeting glioblastoma tumor and cancer stem cell antigens currently in a phase 2 clinical trial of 124 patients with newly diagnosed glioblasto-ma, and recommended that the company continue the trial to completion. The company anticipates that the phase 2 trial should be completed by the end of 2013.[xix]
Northwest Biotherapeutics is also developing a dendritic cell vaccine called DCVax®-L. DCVax-L is currently the subject of a phase 3 trial expected to enroll 312 patients with newly diagnosed glio-blastoma. According to ClinicalTrials.gov, the study started in December 2006 and the primary objec-tive of this study is to compare progression free survival (PFS) from time of randomization between patients treated with DCVax-L and control patients. The secondary objective is to compare overall survival. Estimated primary completion date is September 2014.[xx]
Release the Brake; Step on the Gas
In view of the fact that blocking immune checkpoint proteins disables a brake preventing the immune system from attacking cancer cells, it is only logical to consider synergistic combination ap-proaches with agents that stimulate the immune system, acting like a gas pedal. Such combination approaches with checkpoint inhibitors and cancer vaccines are currently underway with encouraging early results.
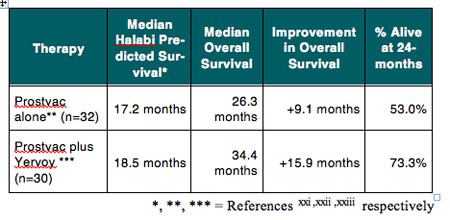
For example, results of a phase 1 dose escalation study provide proof of concept that the combination of the immune checkpoint inhibitor Yervoy with Prostvac®, a therapeutic vaccine candidate for advanced prostate cancer, is feasible and might enhance the clinical efficacy of the combination treatment. Prostvac is the subject of an ongoing pivotal phase 3 trial by Bavarian Nordic A/S (BA-VA.CO, BVNKF), which includes a prespecified interim analyses of data to evaluate whether the trial should continue as planned or potentially be stopped early for efficacy. As shown in Table 3, the median overall survival of 34.4 months for the 30 patients treated raises the possibility that the combina-tion Yervoy and Prostvac might result in prolonged overall survival. Importantly, the use of Prostvac did not seem to exacerbate the immune-related adverse events associated with Yervoy.
In view of the competitive landscape for checkpoint inhibitors, the potential to combine these agents with cancer vaccines and other synergistic approaches could serve as a key differentiator in certain diseases. Accordingly, it would not be surprising to see strategic transactions develop among owners of “gas pedals” and those who control a “brake release.”
Beyond Fighting Cancer
In addition to treating cancer, researchers are exploring immunotherapy approaches in other disease settings with expected near-term catalysts. This includes infectious diseases, allergies, and other areas of unmet medical need.
For example, through the application of one of the company’s novel adjuvant systems, GlaxoSmithKline demonstrated for the first time that a vaccine against a parasite is feasible. Results from a large-scale phase 3 trial showed that the company’s RTS,S malaria vaccine candidate, which contains MPL, QS-21 Stimulon adjuvant, and liposomes, helps protect African infants against malaria.[xxi],[xxii] More data on the longer-term efficacy of the vaccine during 30 months of follow-up after the third dose, and the impact of a booster dose are expected to be publicly available at the end of 2014.[xxiii] The World Health Organization (WHO) has indicated that a policy recommendation for the RTS,S malaria vaccine candidate is possible as early as 2015.
In other infectious diseases, during the fourth quarter of 2013 Agenus is expected to announce results from the company’s phase 2 randomized, double-blind, multi-center study for HerpV, a recombinant "off-the-shelf" therapeutic vaccine candidate for the treatment of genital herpes in herpes simplex virus-2 (HSV-2) positive patients.[xxiv] Similar to the aforementioned programs by Glax-oSmithKline, HerpV contains Agenus' QS-21 Stimulon adjuvant.
In September 2011, positive results from a randomized, four-arm phase 1 study of HerpV were published in the peer-reviewed journal Vaccine.[xxv] All patients who were evaluable for immune response and received HerpV showed a statistically significant CD4+ T cell response (100%; 7/7) to HSV-2 antigens as detected by IFNy Elispot, and the majority of those patients demonstrated a CD8+ T cell response (75%; 6/8).
In May 2013, Merck announced that the Biologics License Application (BLA) for its investigational ragweed pollen (Ambrosia artemisiifolia) sublingual allergy immunotherapy tablet had been accepted for review by the FDA. On March 27, 2013, Merck announced that the FDA had also accepted the BLA for its investigational Timothy grass pollen (Phleum pratense) sublingual allergy immunotherapy tablet. Merck expects the FDA’s review for both to be completed in the first half of 2014.[xxvi]
Merck has partnered with ALK-Abelló A/S (ALK-B.CO) to develop its sublingual, dissolvable aller-gy immunotherapy tablets for ragweed pollen, timothy grass pollen and house dust mite in North America. The tablets are designed to generate an immune response to help protect against the targeted allergen. In June 2013, ALK announced positive top-line results from the company’s pivotal phase 3 MERIT trial conducted with an immunotherapy tablet against house dust mite allergy. The trial was designed to form a pivotal part of ALK's submission of a registration application in Europe, which is expected to occur in 2014.[xxvii]
Conclusion
As of January 1, 2009, it was estimated that more than 12.5 million living people in the United States alone had received a cancer diagnosis.[xxviii] The treatments that many of these patients receive, such as chemotherapy and radiation therapy, often cause more fear and anxiety than the diagnosis itself. This is due to concerns over side effects, such as nausea, fatigue, hair loss and many others.
In contrast, cancer immunotherapy—in particular cancer vaccines—offers the promise of less tox-ic and more efficacious therapeutic alternatives for patients. Researchers have learned more about the body’s immune system over the past 15 years, resulting in over a dozen important regulatory milestones during this period. The commercial availability of these agents facilitates novel combina-tion approaches that may provide even greater insight into the body’s immune system.
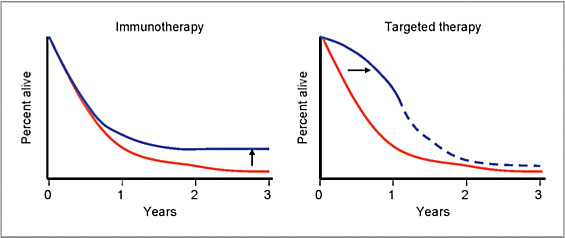
As evidenced in a phase 3 clinical study with Yervoy, recently introduced cancer immunotherapy agents offer the prospect of cure in some patients. The late plateau (or tail) in the survival curve for Yervoy, representing long-term responders who remain relapse free for years, is truly indicative of the disruptive potential for the field of cancer immunotherapy (Chart 3).[xxix]
As a result of these advances, the era of skepticism over use of the body’s immune system to effectively treat cancer has officially come to an end. We are now firmly in the early stages of the cancer immunotherapy revolution. The $12.9 billion added to Bristol-Myers’ market capitalization during the time period surrounding the ASCO 2013 Annual Meeting only reinforces that Wall Street is indeed shifting from pessimism to optimism for the field of cancer immunotherapy.
Chart 3: Effects of immunotherapy and targeted therapy on melanoma survival curves
Adding to the recent momentum, the Cancer Research Institute (CRI), a leading nonprofit organization in cancer immunotherapy research, recently designated June as Cancer Immunotherapy Awareness Month.[xxx] The annual one-month celebration is designed to highlight important advances in the treatment of cancer thanks to scientific breakthroughs in harnessing our immune system’s power to fight all types of cancer.
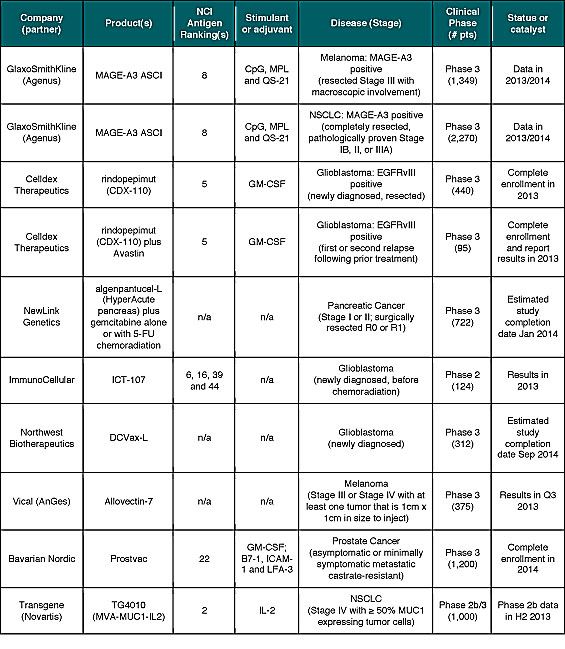
As shown in Table 4, there are at least ten cancer vaccine programs in mid- and late-stage development with important catalysts expected during the balance of 2013 and into 2014. Importantly, several of these newer products seem to work better and address the limitations of prior cancer vaccine approaches. Positive results with any of these studies may help break investor’s “immune tolerance” to this exciting and important segment of the cancer immunotherapy market.
In addition to prospects for positive clinical outcomes, all but two of the programs referenced in Table 4 are “off-the-shelf” cancer vaccines that should offer improved commercial and/or financial prospects, such as revenue and cost of goods sold (COGS), when compared to autologous approaches. This has been a major criticism of the first cancer vaccine product, Provenge, for which Dendreon is aiming to reduce COGS to below 50% of net product revenue in the third quarter of 2013.[xxxi] In contrast to Yervoy, which generated $229 million in worldwide sales during the first quarter of 2013, net product revenue for Provenge in the United States during the same period was $67.6 million.
Table 4. Select Cancer Immunotherapy Programs Mentioned in this Report
Legal Disclaimer
This report has been prepared by MDB Communications LLC from information sources believed to be reliable. MDB Communications expressly disclaims any and all liability which may be based on such information, errors therein or omissions therefrom, or in any other written or oral communication related thereto.
This report is for informational purposes only and should not be construed as investment advice or as any recommendation of a transaction. MDB Communications is not soliciting any action based upon it. Specifically, this material is not, and is not to be construed as, an offer to buy or sell any security or other financial instrument referred to herein. To the extent any of the information in this report may be deemed to be investment advice, such information is impersonal and not tailored to the investment needs of any specific person. Furthermore, this report is not intended for distribution to, or use or access by, any person in any jurisdiction where such distribution, use or access would be contrary to applicable law or regulation or which would subject MDB Communications to any additional registration or licensing requirements.
MDB Communications is not a broker/dealer registered with FINRA (formerly known as NASD) or any other regulatory agency and in the performance of the communications services described in the firm’s website, MDB Communications shall not be considered to be acting in any broker/dealer or underwriting capacity and therefore MDB Communications will not receive any compensation as such.
With respect to any companies discussed in this report, MDB Communications or its affiliates may provide, may have provided, or may seek to provide communications services to such issuers that could affect the objectivity of such information. MDB Communications or such affiliates do not and will not hold positions or otherwise trade or effect transactions in the securities of the companies discussed herein and do not accept payment of any fees in company stock or any form of security.
The materials provided herein belong exclusively to MDB Communications. They may not be cop-ied, reproduced, republished, posted, transmitted or redistributed in any way, without the prior written consent of MDB Communications. No information herein may be modified or used for any other purpose without the prior written consent of MDB Communications.
Author Biography
Michael D. Becker, president and founder of MDB Communications LLC, has more than 15-years of experience as a serial entrepreneur, C-level industry executive, drug developer, Wall Street securities analyst and registered financial advisor. An early champion for the field of cancer immunotherapy, he has authored original articles on medical, financial, communications, marketing, and regulatory topics relevant to the biotechnology, pharmaceutical, and medical device industries and is widely quoted by the press.
Before establishing MDB Communications LLC in June 2013, Mr. Becker was founder and man-aging partner of the successful consulting firm MD Becker Partners LLC from 2008-2013. He also previously served as president, chief executive officer, and member of the Board of Directors for several publicly traded biotechnology companies including commercial-stage Cytogen Corporation (acquired by EUSA Pharma) and development-stage VioQuest Pharmaceuticals, Inc.
Mr. Becker held positions of increasing responsibility prior to being appointed President and CEO of Cytogen in 2002, including Vice President of Business Development, Industry Relations, Investor Relations and Chief Executive Officer of AxCell Biosciences, a subsidiary of Cytogen focused on signal transduction pathways. During his tenure at Cytogen, Mr. Becker raised in excess of $130 million in new capital through both public offerings and private placements and in-licensed Caphosol©, a topical oral agent and prescription medical device for the treatment of oral mucositis and xerosto-mia.
Prior to joining Cytogen, Mr. Becker was with Wayne Hummer Investments LLC, a Chicago-based regional brokerage firm, where he held senior positions as a biotechnology securities analyst, financial advisor and portfolio manager. He was also the founder and Executive Editor of Beck on Biotech, a monthly biotechnology investment newsletter published from July 1998 through March 2001. Mr. Becker was previously with Kidder, Peabody & Co., Gruntal & Co., LLC, and Kemper Securities. He has previously held the following financial licenses: Series 7 (Registered Representative), Series 16 (Securities Analyst), Series 63 (Uniform Securities Agent) and Series 65 (Reg-istered Investment Advisor).
Mr. Becker plays an active leadership role as an advocate for the biotechnology industry. He is past Chairman and member of the board of trustees with BioNJ, which is New Jersey’s trade association for biotechnology companies, and is currently a member of BioNJ. He placed as a biotechnology/life sciences finalist for the Ernst & Young Entrepreneur of the Year award in both 2004 and 2005 and was listed in BusinessWeek’s “CEO’s 40 and Under” article in December 2006. Mr. Becker is also a lifestyle event and portrait photographer working both on location and in the studio. He is a member of the National Press Photographers Association (NPPA) and Professional Photographers of America (PPA). He attended DePaul University in Chicago, Illinois.
References
[i] Company press release “Roche delivers strong 2012 results” from January 30, 2013
[ii] Company press release “Bristol-Myers Squibb Reports First Quarter 2013 Financial Results” from April 25, 2013
[iii] N Engl J Med. 2013 Jun 2. [Epub ahead of print] Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Jo-seph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elas-saiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A.
[iv] N Engl J Med. 2013 Jun 2. [Epub ahead of print] Nivolumab plus Ipilimumab in Advanced Melanoma. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M.
[v] Lancet. 2008 Jul 12;372(9633):145-54. doi: 10.1016/S0140-6736(08)60697-2. Epub 2008 Jul 3. An adjuvant autolo-gous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, Isakov L, Flanigan R, Figlin R, Gupta R, Escudier B; C-100-12 RCC Study Group.
[vi] J Clin Oncol. 2008 Feb 20;26(6):955-62. doi: 10.1200/JCO.2007.11.9941. phase III comparison of vitespen, an autolo-gous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Sri-vastava PK; C-100-21 Study Group.
[vii] Lancet. 1999 Jan 30;353(9150):345-50. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG Jr, Pinedo HM.
[viii] NCI Understand-ing Cancer Series - The Immune System: http://cancer.gov/cancertopics/understandingcancer
[ix] J Natl Cancer Inst. 2011 Oct 19;103(20):1488-9. Epub 2011 Sep 26. Oncology micro-cap stocks: caveat emptor! Feuerstein A, Ratain MJ.
[x] Company press release “GSK delivers Q1 2013 core EPS of 26.9p and dividend of 18p” from April 24, 2013
[xi] Clin Cancer Res. 2009 Sep 1;15(17):5323-37. doi: 10.1158/1078-0432.CCR-09-0737.
The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM.
[xii] Expert Rev Vaccines. 2007 Oct;6(5):723-39. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achieve-ments and perspectives. Garçon N, Chomez P, Van Mechelen M.
[xiii] Front Biosci. 2000 Jan 1;5:D213-31. Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Smith JS, Jenkins RB
[xiv] Company press release “Celldex Reports First Quarter 2013 Financial Results” from May 2, 2013
[xv] Company press release “Agenus Announces Initiation of Enrollment in NCI-Sponsored Randomized Trial of Prophage G-200 Vaccine With Avastin in Treatment of Brain Tumors” from May 22, 2013
[xvi] Transgene corporate website (http://www.transgene.fr) accessed July 7, 2013
[xvii] Cancer Sci. 2013 Mar;104(3):282-90. doi: 10.1111/cas.12084. Epub 2013 Feb 6. Role of α-gal epitope/anti-Gal antibody reaction in immunotherapy and its clinical application in pancreatic cancer.
Tanemura M, Miyoshi E, Nagano H, Eguchi H, Taniyama K, Kamiike W, Mori M, Doki Y.
[xviii] Company press release “Vical Reports First Quarter 2013 Financial Results and Progress in Key Development Programs” from May 9, 2013
[xix] Company press release “ImmunoCellular Therapeutics Announces Recommendation of Data Monitoring Committee to Continue ICT-107 phase II Trial Following Interim Analysis” from June 7, 2013
[xx] ClinicalTri-als.gov http://www.clinicaltrials.gov/ct2/show/NCT00045968
[xxi] N Engl J Med. 2011 Nov 17;365(20):1863-75. doi: 10.1056/NEJMoa1102287. Epub 2011 Oct 18. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al., et al.
[xxii] N Engl J Med. 2012 Dec 13;367(24):2284-95. doi: 10.1056/NEJMoa1208394. Epub 2012 Nov 9. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, et al., et al.
[xxiii] Company press release “RTS,S malaria candidate vaccine reduces malaria by approximately one-third in Afri-can infants” from November 9, 2012
[xxiv] Company press release “Agenus Reports First Quarter 2013 Financial Results” from April 24, 2013
[xxv] Vaccine. 2011 Nov 3;29(47):8520-9. doi: 10.1016/j.vaccine.2011.09.046. Epub 2011 Sep 21. Safety and immuno-genicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Wald A, Ko-elle DM, Fife K, Warren T, Leclair K, Chicz RM, Monks S, Levey DL, Musselli C, Srivastava PK.
[xxvi] Company press release “Merck Announces FDA Acceptance of Biologics License Application for Investigational Ragweed Pollen Sublingual Allergy Immunotherapy Tablet” from May 08, 2013
[xxvii] Company press release “ALK announces positive outcome of pivotal phase III trial with new allergy immuno-therapy tablet against house dust mite allergy” from June 19, 2013
[xxviii] Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2009, National Cancer Institute. Bethesda, MD, based on November 2011 SEER data submission, posted to the SEER Web site, 2012 accessed at http://seer.cancer.gov/csr/1975_2009/ on May 1, 2012.
[xxix] Clin Cancer Res. 2012 Jan 15;18(2):336-41. doi: 10.1158/1078-0432.CCR-11-2323. Epub 2011 Dec 5. New chal-lenges in endpoints for drug development in advanced melanoma. Ribas A, Hersey P, Middleton MR, Gogas H, Flaherty KT, Sondak VK, Kirkwood JM.
[xxx] Cancer Research Institute website: http://www.cancerresearch.org/get-involved/cri-events/cancer-immunotherapy-awareness-month
[xxxi] Company press release “Dendreon Announces First Quarter 2013 Results” from May 9, 2013
Michael Becker
The Life Science Digest


























































