Alto Neuroscience Inc. (ANRO:NYSE) recapped events related to its investigative drug pipeline and announced its Q3/25 financial numbers, noted a news release earlier last month.
"Alto reported Q3/25 results marked by continued financial, clinical, and regulatory progress across its late-stage programs," H.C. Wainwright & Co. (HCW) Analyst Patrick Trucchio wrote in a November 14 research report.
This California, U.S.-based firm is developing drug candidates for psychiatric and neurological disorders, but in doing so, is employing a precision versus one-size-fits-all approach. In other words, the company is using patients' specific neurobiology to develop personalized, highly effective treatment options.
Here are the latest developments with four of the clinical-stage, investigative drugs in the company's pipeline, according to the news release:
ALTO-207: This is a fixed-dose combination of pramipexole, a dopamine D3-preferring D3/D2 agonist with antidepressant effects, and ondansetron, an antiemetic selective 5-HT3 receptor antagonist, which Alto is developing for patients with treatment-resistant depression (TRD), a significant unmet need.
After a successful meeting with the U.S. Food and Drug Administration (FDA) last month about this program and based on the agency's feedback, Alto is accelerating ALTO-207's development. The biopharma is on track to launch a Phase 2b trial in H1/26 and, after alignment with the FDA on the planned study design, will commence a Phase 3 trial by early 2027. The Phase 3 will run concurrently with the Phase 2b.
ALTO-101: This is a brain-penetrant PDE4 inhibitor in a transdermal formulation that Alto is developing to treat cognitive impairment in schizophrenia (CIAS), a US$1 billion-plus (US$1B-plus) opportunity with no approved treatments. Transdermal administration should deliver the desired brain effects shown with the oral formulation, without the adverse effects and intolerability often associated with PDE4 inhibitors.
In October, the FDA granted ALTO-101 Fast Track designation for CIAS. Also, a blinded pharmacokinetic (PK) analysis of samples from the Phase 2 trial's first cohort showed all of them to be PK positive, indicating Alto's enhanced execution efforts are effective. Alto determined that the strongest objective electroencephalogram (EEG) biomarker for distinguishing patients with schizophrenia from patients without it is prospective replication and validation of theta ITC (theta-band intertrial coherence). The firm's analysis demonstrated a statistically significant correlation between theta ITC and cognitive performance as well. Alto is continuing to enroll patients in its Phase 2 proof-of-concept trial of ALTO-101 in CIAS. Topline data are slated for release in Q1/26.
ALTO-300: This is an oral, small molecule designed to act as a melatonin agonist and a 5-HT2C antagonist, which Alto is developing at 25 milligrams as an adjunctive treatment in the U.S. for patients with major depressive disorder (MDD) characterized by an EEG biomarker.
The Phase 2b adjunctive MDD trial of ALTO-300 is still in the enrollment phase. Data are anticipated in mid-2026.
ALTO-100: This is a first-in-class, oral small molecule believed to work through enhancing neuroplasticity, which Alto is developing to treat bipolar depression (BPD) characterized by a cognitive biomarker.
A blinded PK analysis of samples from the first cohort in the ongoing Phase 2b study showed that 96% of them qualified as PK positive. Alto is still enrolling patients in the Phase 2b BPD trial, slated to read out in H2/26.
In other recent news, the company closed a private investment in public equity (PIPE) that generated gross proceeds of US$50 million (US$50M). This boosted Alto's cash position as of October 31 to US$184.2M, an amount sufficient to fund planned operations into 2028. At Q3/25's end, the biopharma had US$138.3M in cash, cash equivalents, and restricted cash, compared to US$168.7M a year before.
As for its other Q3/25 financial results, Alto posted a net loss of US$14.2M , an improvement over its US$16.8M net loss a year earlier. Research and development (R&D) expenses and general and administrative (G&A) expenses were both lower in Q3/25 than in Q3/24, at US$10.5M versus US$13.1M and at US$4.4M compared to US$5.8M, respectively.
What Makes ANRO Different?
In addition to a strong cash position, here are three other reasons to invest in Alto Neuroscience now, according to various analysts.
The biopharma differentiates itself from other companies in the space with its approach to drug development. With its proprietary Precision Psychiatry Platform, Alto measures brain biomarkers — through analysis of EEG activity, neurocognitive assessments, and wearable data, for example — and other metrics to better identify which patients are more likely to respond to its drug candidates.
In doing so, the firm is addressing two challenges in psychiatric drug development that historically have resulted in investigative drug failures, and they are high placebo response rates and heterogeneous patient populations. Alto's approach could improve success rates dramatically. Thus far, compliance rates in Alto's trials have been high, between 96% and 100%, suggesting the firm's approach is working.
Alto has a near-term value driver with ALTO-207, which could reach the market a year earlier than expected with the accelerated development path. The drug could be granted FDA approval in 2029-2030. The target market for ALTO-207 is the same as for Johnson & Johnson's (JNJ:NYSE) Spravato, which generates about US$1.8B a year. Alto projects that ALTO-207 could generate US$485–600M in revenue by 2031.
Alto currently has multiple shots on goal with four independent, diverse clinical programs (ALTO-100, ALTO-101, ALTO-207, and ALTO-300), each with different mechanisms and biomarkers, each addressing a specific large unmet need in psychiatry and neurology. Each has a data readout coming up in the near-to-medium term.
Sector Seeing 14.9% CAGR
Precision psychiatric drug development is a new approach that is gaining traction, according to an October 13 article on pharmexec.com. It involves using patient data like genetics, biomarkers, and neurobiology to create more targeted and effective treatments, ones with better outcomes and faster recovery, for psychiatric disorders.
Biomarkers are "characteristics measured as an indicator of normal biological processes, pathogenic processes or biological responses to an exposure or interventions, including therapeutic interventions," according to a June 19, 2025, article in Molecular Psychiatry. "The goal of precision psychiatry cannot be achieved without biomarkers."
Examples of biomarkers are neuroimaging, such as EEG and functional magnetic resonance imaging; genomic data like polygenic risk scores; protein and metabolic markers; physiological signals; data from wearable devices and other digital tools that can provide objective, continuous data on a patient's condition, noted a May 2025 article in the International Journal of Neuropsychopharmacology.
Precision psychiatry segments broader patient populations into more specific subsets, for example, MDD patients with insomnia, MDD patients with anhedonia and suicidal ideation, and patients with suicidal treatment-resistant BPD. Drugs or treatments then may be tailored to a narrower group, or subset.
"For biopharma companies, navigating this evolving landscape presents substantial opportunities to differentiate themselves in a complex therapeutic area," the PharmExec article noted.
The global precision psychiatry market size is forecasted to expand at a 14.9% compound annual growth rate between 2025 and 2033, reaching US$2.9B in value from US$825.6M, according to a Grand View Research report. Growth drivers include the increasing prevalence of mental health disorders, growing demand for personalized treatment approaches, advancements in genomics and biomarker research, and rising adoption of artificial intelligence (AI) and digital health tools to improve diagnostic accuracy and treatment outcomes.
In terms of geography, North America accounted for 41% of the global precision psychiatry market in 2024. Grand View attributed this to the region's high spending on healthcare, strong private and public investment in R&D, and rapid clinical adoption of genomics and digital therapeutics.
The Catalysts: Trials and Data
Alto has several upcoming events in the next 2-plus years, according to the company. Specifically, ALTO-101 Phase 2 data in CIAS are expected to be released in Q1/26. The release of ALTO-300 Phase 2b data in MDD and the start of the ALTO-207 Phase 2b study in TRD are expected to happen in mid-2026. Results from the Phase 2b ALTO-100 trial in BDP are slated for release in H2/26.
Further out, Alto is expected to launch the Phase 3 trial of ALTO-207 in TRD in early 2027. Subsequently, the biopharma will release data from this and the Phase 2b ALTO-207 study in the same indication in 2027 and 2028.
Analysts Bullish on Stock
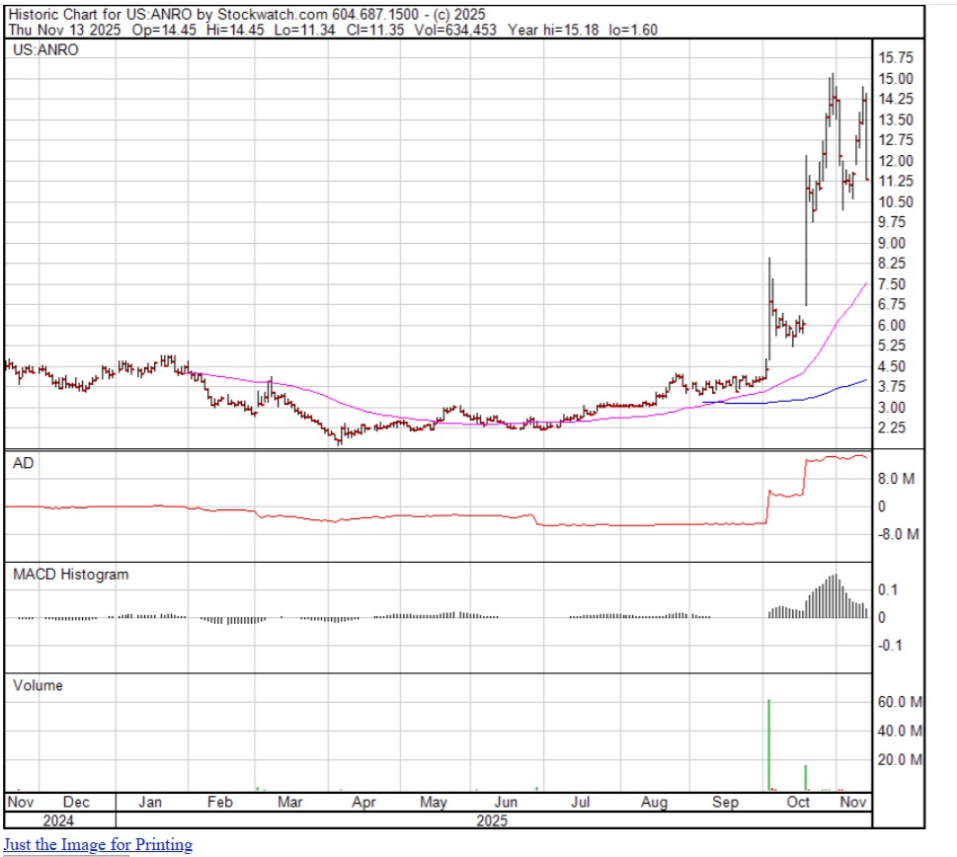
BTIG Analyst Dr. Thomas Shrader reported in a November 17 research report that he initiated coverage on Alto Neuroscience with a Buy rating and a US$27 target price, implying over 96% upside from ANRO's closing price that day of US$13.51.
Shrader highlighted Alto's personalized psychiatric approach to drug development. He explained that to come up with biomarkers to use for its clinical programs, the biopharma "casts a wide net" to find potential biomarkers, then does the necessary work to determine which ones really are predictive of clinical results. The biomarkers Alto selects must be applicable outside of academic institutions.
"This precision approach should lead to a higher likelihood of clinical success during development and a more compelling final label for successful drugs," Shrader wrote.
The analyst pointed out the many possible stock-boosting events coming up for Alto and how the ALTO-300 Phase 2b data readout is the "biggest." He wrote, "The story is rich in meaningful catalysts with four major readouts over the next two years, including three Phase 2b trials that each could be meaningful inflection points for the stock."
He noted that the ALTO-300 program involves improving a drug already approved in the European Union, agomelatine, and that ALTO-100 and ALTO-101 have broader applicability beyond CIAS and BPD, respectively.
On November 14, H.C. Wainwright's Trucchio reiterated his Buy rating and a US$50 target price. The closing price at the time of the report was US$12.11.
He wrote that Alto and the FDA agreeing on an accelerated development path for ALTO-207, Alto receiving Fast Track designation for ALTO-101, and near-perfect patient compliance in ongoing trials all "underscore operational rigor and clinical consistency across Alto's biomarker-guided portfolio."
HCW believes ALTO-207 in TRD has blockbuster potential if pivotal data replicate the Phase 2a signal and estimates its worth at US$25/share. HCW views ALTO-300 in MDD as having a clear safety advantage over existing atypical antipsychotic adjuncts, estimates its peak annual revenues of US$2.5B-plus, and calculates its worth at US$20/share. HCW estimates ALTO-100 in BPD generating peak annual revenues of US$1B-plus in BPD and being worth US$3/share.
Next year will be a turning point for Alto, according to Trucchio, who wrote, "Capital strength, regulatory momentum, and multiple upcoming catalysts position Alto Neurosciences for an inflection point in 2026."
After Alto released its Q3/25 update, in a November 13 research report, Stifel Analyst Paul Matteis maintained his Buy rating but raised his target price on the biopharma. The new target implies a 139% uplift from ANRO's November 17 closing price of US$13.80.
 In an October 20 research report, William Blair Analyst Dr. Myles Minter maintained his Outperform rating and no target price on the biopharma with an attractive risk/reward profile. He highlighted Alto's US$50M private placement, positive FDA meeting, and decision to accelerate the Phase 3 ALTO-207 in TRD, "a major market with significant unmet need."
In an October 20 research report, William Blair Analyst Dr. Myles Minter maintained his Outperform rating and no target price on the biopharma with an attractive risk/reward profile. He highlighted Alto's US$50M private placement, positive FDA meeting, and decision to accelerate the Phase 3 ALTO-207 in TRD, "a major market with significant unmet need."
As for Alto's prospects, he wrote, "Ultimately, we see a diversified asset pipeline, mixed with a strong cash position through at least four clinical readouts, as increasing the likelihood of eventual clinical win(s) and the room for a several-fold price appreciation from current levels."
Andrew Tsai, equity analyst at Jefferies, highlighted in an October 20 research report that, in the bigger picture, Alto "is looking to disrupt the central nervous system treatment paradigm by leveraging precision psychiatry (biomarkers) to maximize positive study outcomes." Alto's clinical programs are independent and feature different compounds and biomarkers, but they all share the same data-science approach, the analyst wrote. He noted the 96–100% patient compliance rates in the ongoing Phase 2 studies of ALTO-101 and ALTO-100.
Looking ahead, Tsai wrote, "The stock could move higher if we see convincing efficacy in any of ANRO's four-plus, placebo-controlled Phase 2 data sets in 2026-2027. The analyst has a Buy rating on Alto and a target price implying a 9% gain from its November 17 closing price.
Wedbush Analyst Dr. Laura Chico wrote in an October 21 research report that ALTO-207 for TRD could launch in the U.S. conservatively in early 2029 based on Alto's provided timeline. Chico has a Buy rating on Alto. Her target price is outdated, as the biopharma already climbed past it.
Ownership and Share Structure1
Six insiders own 6.52% of Alto, including Founder, President, and Chief Executive Officer Dr. Amit Etkin with 3.88%. Numerous institutions have 65.33%. The Top 3 institutional shareholders are Alpha Wave Global LP with 11.94%, Perceptive Advisors LLC with 6.4% and Commodore Capital LP with 5.32%. Retail investors hold the rest.
Alto has 31.07 million shares outstanding. Its market cap is US$376.21M. Its 52-week range is US$1.60–15.18 per share.
| Want to be the first to know about interesting Biotechnology / Pharmaceuticals investment ideas? Sign up to receive the FREE Streetwise Reports' newsletter. | Subscribe |
Important Disclosures:
- As of the date of this article, officers, contractors, shareholders, and/or employees of Streetwise Reports LLC (including members of their household) own securities of Alto Neuroscience Inc.
- Doresa Banning wrote this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor.
- This article does not constitute investment advice and is not a solicitation for any investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Each reader is encouraged to consult with his or her personal financial adviser and perform their own comprehensive investment research. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company.
- This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.
For additional disclosures, please click here.
1. Ownership and Share Structure Information
The information listed above was updated on the date this article was published and was compiled from information from the company and various other data providers.