We did very well with Talaris as a takeover target. My next pick here is Nkarta Inc. (NKTX:NASDAQ).
Recent Price: US$3.20
Shares Outstanding: 47 million
NKTX is a takeover target, selling below cash value as well as a potential short squeeze. As of September 30, 2023, Nkarta had cash, cash equivalents, restricted cash, and investments of US$278.4 million. So, the stock is trading well below the cash value of about $5.90.
A colleague of mine analyzed the institutional share ownership. He summarized that new owners bought 20% of the company in October, and around 10% of the company was shorted to them. He figures that 80% of the stock is unlikely to sell. With 14% of the company shorted, there's a high likelihood of a short squeeze to a cash value of around US$5.65/share, when we consider some cash burn since September 30.
It is also a takeover target for a company like Vertex Pharmaceuticals Inc. (VRTX:NASDAQ) because both Vertex and Nkarta are partnered and use the same CRISPR Therapeutics, Inc. (CRSP: NASDAQ) technology, a type of cell therapy:
- Vertex at US$407 per share has a market cap of US$105 billion
- CRISPR at US$62 has a market cap of US$5 billion
- Nkarta at US$3.00 has a market cap of only $141 million
CRISPR's gene therapy treatment got its first FDA approval. Vertex has popped higher after news on December 8 that Vertex Pharmaceuticals and CRISPR Therapeutics announced that the U.S. Food and Drug Administration (FDA) has approved CASGEVY™ (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 genome-edited cell therapy, for the treatment of sickle cell disease (SCD) in patients 12 years and older with recurrent vaso-occlusive crises (VOCs).
This approval means that for the first time, approximately 16,000 patients with SCD may be eligible for a durable one-time therapy that offers the potential of a functional cure for their disease by eliminating severe VOCs and hospitalizations caused by severe VOCs.
"CASGEVY's approval by the FDA is momentous: it is the first CRISPR-based gene-editing therapy to be approved in the U.S. As importantly, CASGEVY is a first-in-class treatment that offers the potential of a one-time transformative therapy for eligible patients with sickle cell disease," said Reshma Kewalramani, M.D., Chief Executive Officer and President of Vertex. "I want to convey my deepest gratitude to the patients and investigators whose trust in this program paved the way for this landmark approval."
"When our company was founded, we had a vision to translate CRISPR technology into multiple breakthrough therapies. So, this U.S. approval of the first-ever medicine using CRISPR gene editing is breathtaking and a truly humbling moment for me personally and for the whole organization," said Samarth Kulkarni, Ph.D., Chairman and CEO of CRISPR Therapeutics.
CRISPR Therapeutics (Nasdaq: CRSP) is a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases. In early December, they provided an update on its immuno-oncology pipeline of CRISPR/Cas9 gene-edited allogeneic chimeric antigen receptor (CAR) T cell product candidates. And, more important expand into Autoimmune Diseases.
Nkarta is also partnered with CRISPR, but their treatment is at an earlier stage of the FDA approval process. Nkarta is a clinical-stage biotechnology company advancing the development of allogeneic, off-the-shelf natural killer (NK) cell therapies. By combining its cell expansion and cryopreservation platform with proprietary cell engineering technologies and CRISPR-based genome engineering capabilities, Nkarta is building a pipeline of future cell therapies engineered for deep therapeutic activity and intended for broad access in the outpatient treatment setting.
The company has been focusing its NKX101 therapy on acute myeloid leukemia (AML). In June 2023, Nkarta reported updated clinical data from its Phase 1 clinical trial evaluating NKX101 in patients with relapsed or refractory (r/r) AML. In patients that received NKX101 after LD comprising fludarabine and cytarabine (Flu/Ara-C), 4 of 6 achieved CR/CRi. Flu/Ara-C LD is expected to be the basis of NKX101 development moving forward. Nkarta plans to present a poster at the American Society of Hematology annual meeting in December 2023 with follow-up data on the six patients from the June 2023 report that received NKX101 after Flu/Ara-C LD.
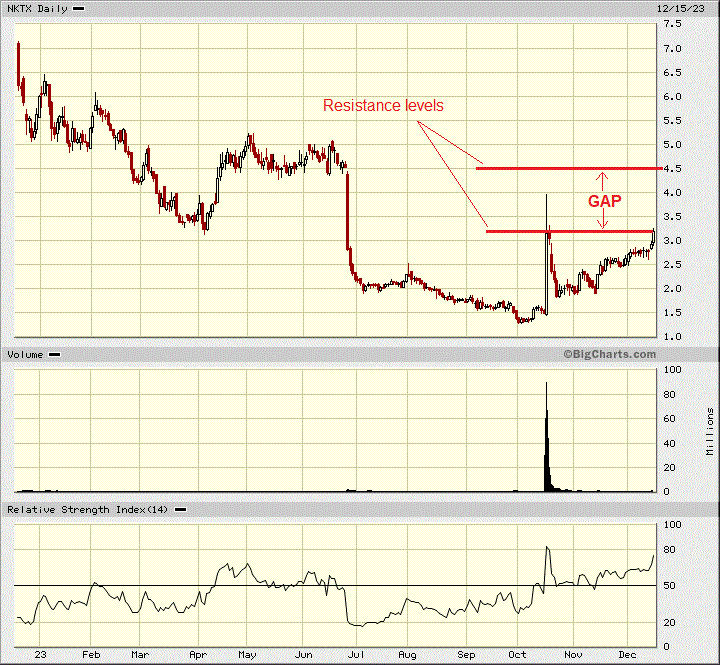
In the short term, the stock is overbought and approaching the first resistance area. However, this hopefully provides liquidity for buyers.
Stock will most often move up to close previous gaps on the way down, and that is what I expect NKTX will do and move up between US$4.50 and US$5.00. We could do much better if there is a takeover offer and/or positive news on their therapy treatment.
| Want to be the first to know about interesting Biotechnology / Pharmaceuticals investment ideas? Sign up to receive the FREE Streetwise Reports' newsletter. | Subscribe |
Important Disclosures:
- [Ron Struthers]: I, or members of my immediate household or family, own securities of: [Nkarta Inc.]. I determined which companies would be included in this article based on my research and understanding of the sector.
- Statements and opinions expressed are the opinions of the author and not of Streetwise Reports or its officers. The author is wholly responsible for the validity of the statements. The author was not paid by Streetwise Reports for this article. Streetwise Reports was not paid by the author to publish or syndicate this article. Streetwise Reports requires contributing authors to disclose any shareholdings in, or economic relationships with, companies that they write about. Streetwise Reports relies upon the authors to accurately provide this information and Streetwise Reports has no means of verifying its accuracy.
- This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company.
- This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.
For additional disclosures, please click here.
Struthers Resource Stock Report Disclosures
All forecasts and recommendations are based on opinion. Markets change direction with consensus beliefs, which may change at any time and without notice. The author/publisher of this publication has taken every precaution to provide the most accurate information possible. The information & data were obtained from sources believed to be reliable, but because the information & data source are beyond the author's control, no representation or guarantee is made that it is complete or accurate. The reader accepts information on the condition that errors or omissions shall not be made the basis for any claim, demand or cause for action. Because of the ever-changing nature of information & statistics the author/publisher strongly encourages the reader to communicate directly with the company and/or with their personal investment adviser to obtain up to date information. Past results are not necessarily indicative of future results. Any statements non-factual in nature constitute only current opinions, which are subject to change. The author/publisher may or may not have a position in the securities and/or options relating thereto, & may make purchases and/or sales of these securities relating thereto from time to time in the open market or otherwise. Neither the information, nor opinions expressed, shall be construed as a solicitation to buy or sell any stock, futures or options contract mentioned herein. The author/publisher of this letter is not a qualified financial adviser & is not acting as such in this publication.